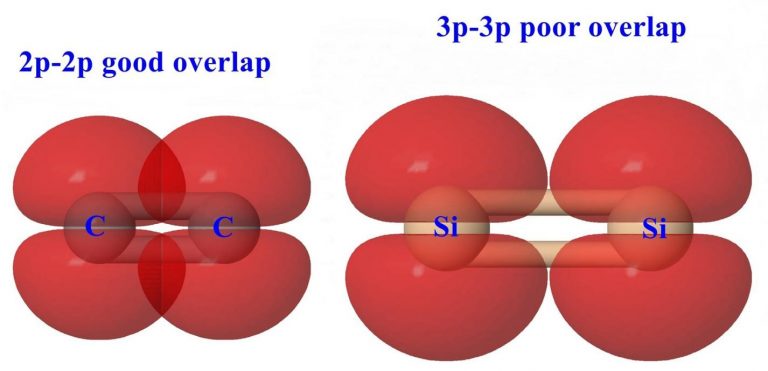
One of the hallmarks of carbon is its ability to form multiple bonds to itself or to other elements (particularly O, N or S). Arguably this feature of carbon is one of the most important reasons for the richness and diversity of organic chemistry. The inability to prepare compounds of the type R2E=ER2 (E = Si, Ge, Sn, P, As, Sb, Bi) has led to the enunciation of the “double bond rule”. According to this rule element having a principle, a quantum number greater than two should not be able to form multiple bonds (pπ-pπ) within themselves or with other elements. In spite of this rule, chemists have persisted with a sense of doggedness to try and prepare to multiply bonded compounds containing heavier p-block elements (principal quantum number greater than two). The year 1981 makes a watershed in these efforts. In a single year, stable compounds such as R2Si=SiR2, R2Si=CR & RP=PR were prepared and structurally characterized. Later this was extended to other systems and ultimately even the compounds with the heaviest main group element, RBi=BiR has also been realized.
This flurry of activity began with the success of Robert West at the University of Wisconsin, Madison, USA. He succeeded in isolating and characterizing the first disilene containing silicon –silicon double bond. His strategy involved a kinetic stabilization of the reactive double bonded system. He reasoned that the reactivity of the double bond in R2Si=SiR2 can be arrested by having a proactive umberalla in the form of sterically encumbered substituents on silicon atoms. He found out that the photolysis of the trisilene Mes2Si(SiMe3)2 (Mes = mesityl) in 3-methylpentane at 77K, first leads to the formation of an intensely blue coloured siylene (Mes2Si:). Raising the temperature leads to the formation of a yellow solution from which the first disilene was isolated. Several disilenes have since been synthesized using a variety of strategies. All of the disilenes are characterized by a shortening of Si-Si bond length.
R. Okazaki and N. Tokitoh developed an efficient steric protecting group, 2,4,6-tris(bis(trimethyl silyl)methyl) phenyl (Tbt). This protecting group has been used to prepare doubly bonded bismuth and antimony compounds.
In dibismuthene the length of the bond is 2.8206(8)Å. This is about 6 % shorter than the Bi-Bi single bond length of 2.990(2) Å found for Ph2Bi-BiPh2. The Bi-Bi-C bond angle is 100.5o.
This leads to the conclusion that multiple bonding is indeed possible in heavier p-block elements.
– Prof. (Dr.) Sunil Verma