Table of Contents
Introduction
In order to understand the functional and precise electronic use of different materials, it is important to understand their energy configurations. Furthermore, understanding the nano functionality of the material gives us an opportunity to manipulate the material’s fundamental properties to our advantage. The manipulation at nanolevel can help us realize different physical and chemical properties that can be appreciated at the macro level. Materials are basically classified into three main categories as conductors, semiconductors and insulators. These materials behave differently under the same conditions, and this is attributed to their differences in energy level configurations. Among the key configurations are the Fermi level and Band Gap.
Fermi Level
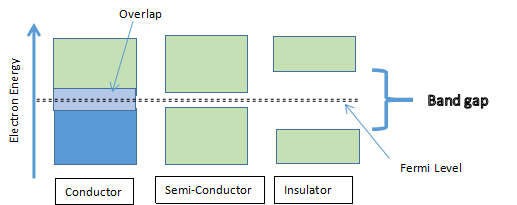
A Fermi level is defined as the highest energy state occupied by electrons in a material when the temperature is at absolute zero. At absolute zero (-273.15 degrees Celsius), the electrons are not in an excited state, but at rest hence free from the influence of external energy. Electrons will generally occupy higher energy levels or orbits than the Fermi level as the temperature is increased above the absolute zero. It also represents the maximum kinetic energy an electron can attain without the help of external energy. The Fermi level, generally, lies in the middle of the bandgap.
Band Gap
An electronic bandgap is defined as the distance of separation between the valence band and the conduction band in a material. The valence band is defined as the outermost orbit of the localization of the electrons in an atom. On the other hand, the conduction band is defined as a de-localized band of energy levels which is located higher than the valence band, in most cases. In the conduction band, the electrons are free to move about due to the absence of orbits. The conductivity of a material is determined by the number of electrons in the conduction band.

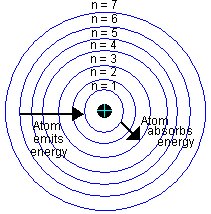
Orbits
Orbits are circular paths at distinct distances from the nucleus. Each orbit is a path of equilibrium for the specific state of energy and is temporally occupied by electrons matching that energy. The electrons then revolve, in the orbit, around the nucleus. The orbits are identified by a principal quantum number, n, which is an integer with increased values with distance from the nucleus. While in any given orbit lower than the conduction band, electrons are in unstable equilibrium and are said to be bound or localized in their orbits. Electrons, being negatively charged, are attracted to the nucleus by coulomb forces between them and the protons, which are positively charged. At temperatures above absolute zero, electrons acquire enough energy against coulomb forces and jump from lower orbits to higher orbits. They fall back to lower orbits once they lose their energy.

Conductors
Conductors are materials that have overlapping valence and conduction bands at absolute zero. Hence conductors have electrons in the conduction band and are able to conduct electricity without the aid from energy outside the atom. The bandgap for conductors is therefore zero.
Semi-conductors
semiconductors have zero electrons in the conduction band at absolute zero. Because their bag gap is small, excitation from external energy causes electrons to jump into the conduction band and are able to conduct electricity. The bandgap for semiconductors can be adjusted by the addition of a controlled amount of a well-selected impurity, in a process called doping. Semi-conductors have two subcategories namely inorganic and organic. The terms valence band and conduction band apply to inorganic while the corresponding terms for organic are highest occupied molecular orbital (HOMO) and lowest occupied molecular orbital (LUMO), respectively.
Insulators
Insulators are materials that have a large bandgap. They have zero electrons in the conduction band at absolute zero. Because their bag gap is large, excitation from external energy fails to promote electrons into the conduction band and hence cannot conduct electricity. For this reason, they are also referred to as bad conductors of electricity.
Conclusion
A brief analysis of the energy configuration of different materials has been made in terms of the Fermi level, bandgap, valence and conduction band.
– by Given Kalonga